Hard capsules are popular in both pharmaceuticals and supplements because they handle a wide range of fills—powders, granules, pellets, or blends—and they scale without changing the dosage form. The challenge is that hard-capsule lines can be sensitive to small shifts. Shell condition affects opening and closing. Fill flow and blend behavior affect weight variation. Humidity, static, and feeding practice often show up quickly as rejects and downtime.
In this guide, capsule manufacturing means producing filled hard capsules using purchased empty shells. The workflow runs from shell selection and controlled handling through fill readiness, blending, hard capsule filling, in-process control, inspection, compliance and troubleshooting basics, and finally primary packaging and storage protection.
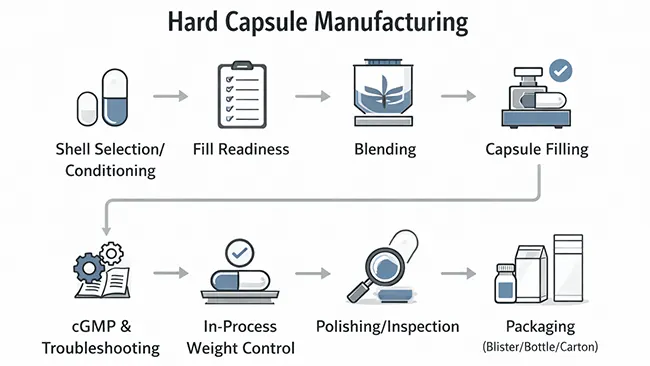
Stage 1 — Capsule Manufacturing: Shell Selection & Incoming Handling (Gelatin vs HPMC)
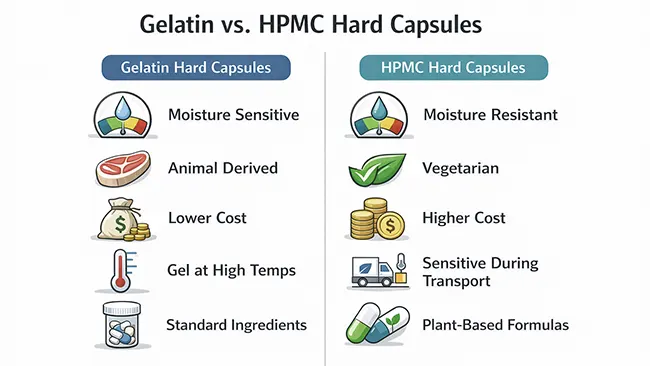
Shell choice sets the limits for the rest of hard capsule manufacturing. For filled hard capsules, the common decision is between gelatin and HPMC (vegetarian) shells.
A practical selection usually comes down to three points:
● Formulation sensitivity: Hygroscopic or humidity-sensitive fills raise the risk of clumping, sticking, and weight drift, so shell choice and handling matter more.
● Market requirements: Vegetarian positioning or region-driven expectations often point to HPMC.
● Cost and supply consistency: Gelatin is widely used and often more cost-effective when handling conditions are stable.
Incoming shells should be treated as a controlled material. Storage conditions during transit, batch-to-batch consistency, and how shells are staged before production can show up later as brittleness, softening, static issues, or closing problems—issues that are easy to blame on the filler but often start upstream.
|
Factor |
Gelatin Capsules |
HPMC Capsules |
|
Common reason to choose |
Cost-effective, widely used |
Vegetarian positioning; broad market fit |
|
Moisture sensitivity |
More sensitive to handling swings |
Often more tolerant, still needs control |
|
Typical best fit |
Stable powders/granules with controlled storage |
Hygroscopic or “tricky” fills; vegetarian requirement |
|
Typical handling risks |
Brittleness (too dry) or softening (too humid) |
Variation by grade; align performance expectations |
|
Dissolution notes |
Common baseline behavior |
Can differ by grade/formulation; verify if critical |
|
Practical takeaway |
Strong choice with stable handling conditions |
Strong choice when formulation/market needs push it |
Even the right shell can fail if it’s handled poorly. In capsule manufacturing, shell conditioning is one of the fastest ways to prevent early-run rejects without changing any filler parameters. Empty hard capsules should be stored under controlled temperature and humidity, then conditioned before production so they reach a stable equilibrium. Skipping this step often leads to avoidable issues at the filler: capsules that crack during handling, caps that don’t seat consistently, or static-driven feeding problems.
Keep the approach simple and repeatable:
● Environment: Use a clean, stable area with controlled humidity. Rapid swings are worse than a steady condition that is slightly off target.
● Staging: Allow shells to acclimate before opening bags or drums. Uneven moisture pickup can create inconsistent behavior across the same lot.
● Exposure control: Minimize open time. Reseal partial containers and keep open shells away from moisture sources and strong airflow.
If rejects rise suddenly at the start of a run and machine settings haven’t changed, check shell condition and handling first.
Many filling problems are material problems. In capsule manufacturing, stable dosing starts with material readiness—flow, moisture, and electrostatics. Before a batch reaches a capsule filling machine, confirm the fill material is ready to flow, feed, and dose. The same formula can behave very differently depending on moisture, particle size distribution, and processing history.
Key readiness points:
● Flowability: Poor flow increases weight variation and can cause bridging. Granulation, flow aids, or particle size adjustment may be needed.
● Moisture content: Too much moisture can cause sticking and buildup; too little can worsen static and dusting. Either can destabilize dosing.
● Particle size and fines: Excess fines increase cohesion and dust; oversized particles can cause inconsistent fill and closing issues.
● Stickiness and electrostatics: Both can drive erratic feeding, especially as run time increases.
If the line looks fine for the first few minutes and then drifts, suspect a slow change in powder behavior—humidity pickup, heat, consolidation in the hopper, or segregation.

Stage 4 — Blending for Uniformity (Prevent Segregation)
Blending is not only about making the mix look uniform. The goal is dose uniformity during feeding, which means preventing segregation after blending—during transfer, hopper loading, and the entire run.
Common drivers of segregation include density differences, wide particle size distribution, vibration during conveying, and long drop heights during transfer. Practical controls that help:
● Blend order and timing: Add lubricants and low-dose components in a way that supports even distribution without overmixing.
● Transfer method: Gentle transfer and consistent paths reduce separation.
● Sampling logic: Confirm uniformity in a way that reflects what the machine sees over time, not only what is easiest to sample.
If early capsules pass and later capsules trend light/heavy, the cause is often segregation in the feed system, not a “mysterious” change in machine performance.
This is the core of capsule manufacturing for filled hard capsules. Equipment level and dosing approach should match batch size, accuracy needs, product behavior, and changeover frequency.
Choosing the machine level
● Manual: Best for trials and very small batches. Low throughput, high labor input, and strong operator influence.
● Semi-automatic capsule filling machine: Useful when you need higher output without full automation complexity. Often a practical step for small commercial volumes.
● Automatic capsule filling machine: Built for higher throughput and repeatable control. This is typically the best fit when downtime and variability cost more than the machine.

When you shortlist suppliers for an automatic capsule filling machine, ask for evidence that the machine stays stable over time, not just speed claims. For example, at Rich Packing, outgoing equipment is commonly verified with a continuous run test plus a high-load verification window before shipment, which helps expose drift and minor stoppages early.
Understanding dosing options
Two dosing mechanisms often discussed are tamping pin and dosator systems. Both aim to deliver a consistent dose into the capsule body, but they can behave differently depending on powder compressibility, cohesion, and target fill weight. The better choice is the one that stays stable with your material and operating window, with manageable cleaning and changeover.
|
Factor |
Manual |
Semi-automatic |
Automatic |
|
Best for |
R&D, samples, trials |
Small commercial, frequent changeovers |
Scale-up, stable output, higher volume |
|
Typical output |
Low |
Medium |
High |
|
Control & repeatability |
Operator-dependent |
Improved, still operator-influenced |
High, consistent run control |
|
Changeover & cleaning |
Simple, slower |
Moderate |
Designed for repeatability, needs discipline |
|
Investment level |
Lowest |
Mid |
Highest |
|
Practical takeaway |
Good to learn the product |
Good bridge for early commercialization |
Best when stability and throughput matter most |
For capsule manufacturing, even with the right capsule filling machine, weight variation can drift as conditions change. In-process control is about catching drift early and correcting it before rejects accumulate.
Common causes include changing powder behavior (humidity pickup, static, consolidation), inconsistent feeding, and gradual buildup on dosing parts. Controls that work in day-to-day production:
● Trend checks: Track weight over time. One spot check can miss drift patterns.
● Material behavior signals: Watch for changes in dusting, flow, or bridging. These often appear before weight failures.
● Adjustment discipline: Change one variable at a time and confirm the impact with a consistent sampling window.
Stable runs come from repeatable control, not “perfect settings.”

After filling, capsules often carry fines or surface dust that can affect appearance and downstream performance. A capsule polishing machine (often paired with dedusting) reduces loose powder that can interfere with packaging and helps deliver a consistent finish.
Inspection focuses on defects that impact quality and line efficiency:
● Cracked or dented capsules (often linked to shell conditioning or mechanical stress)
● Loose caps or poor closing
● Deformed capsules that jam conveyors and packers
● Visible contamination or heavy dusting
● Underfill/overfill outliers that slip through if sampling is too light
This stage protects downstream performance. Packaging problems often start as defects entering the packaging line.
Compliance is built into how the line runs: controlled materials, documented settings, defined cleaning, and clear decisions when something drifts. In hard capsule manufacturing, basic CGMP discipline also makes troubleshooting faster and outcomes more repeatable. (U.S. Food and Drug Administration)
Compliance essentials
● Material control: Supplier qualification, incoming checks, status labeling, and controlled storage.
● Batch records: Document the parameters that affect quality—shell type, blend conditions, machine settings, and in-process results.
● Changeover and cleaning: Define steps and verification, especially when switching products or allergen-sensitive materials.
● Maintenance and calibration: Preventive maintenance reduces drift and unplanned stops.
● Training and deviations: Operators need consistent rules for adjustments, sampling, and escalation.
Troubleshooting patterns (fast checks)
● Weight drifting light/heavy: Check powder behavior first (flow, moisture, segregation), then feeding stability, then buildup or wear on dosing parts.
● Cracking and dents: Re-check shell conditioning and handling, then identify mechanical stress points at transfer interfaces.
● Loose caps/closing failures: Confirm shell size/fit, review humidity effects, and check closing station alignment and wear.
● Sudden reject spikes: Look for upstream changes—new shell lot, environmental swing, hopper refill practice, or an early buildup trend.
A consistent troubleshooting order—material, environment, feeding, then machine settings—reduces guesswork and shortens downtime.
In capsule manufacturing, packaging is part of product protection—not an afterthought—because it determines how well capsules hold up in storage and transport. The right choice of packaging depends on moisture sensitivity, dosing format, and how the product will be handled in distribution.
Blister packaging is often selected when unit-dose presentation, barrier performance, and user convenience matter—typically produced on a blister packaging machine using suitable barrier structures. Bottles are common for multi-dose formats, often combined with desiccants and closure control to manage humidity exposure after opening; this route is frequently paired with a capsule counting and bottling line. For retail-ready presentation and distribution, secondary packaging is commonly handled by a cartoning machine to protect packs in transit and support labeling and traceability.
If capsules soften, become brittle, or show appearance changes on the shelf, investigate the full chain: shell choice and conditioning, fill moisture behavior, and the barrier performance of the selected pack.
Stable output comes from disciplined basics: choose the right shell, handle and condition it consistently, confirm the fill material feeds predictably, blend to resist segregation, and run filling with repeatable in-process control and inspection. With compliance discipline and packaging that matches product sensitivity, capsule manufacturing can scale without chasing drift, rejects, and downtime.
HPMC is often chosen when the fill is moisture-sensitive or when vegetarian positioning is required. The best choice depends on how the full system behaves under your storage conditions and production humidity window.
Common causes include changing powder behavior (moisture pickup, static, consolidation), feed instability, hopper practice, and gradual buildup on dosing parts. Trending weight over time helps detect drift early.
Choose based on stability with your powder, target fill weight, and run window. The mechanism that maintains consistent dosing with manageable cleaning and changeover is usually the better fit.
Shell conditioning and handling are frequent contributors. Over-dry shells can become brittle, while mechanical stress at transfer points can dent or crack capsules. Review shell staging, environment, and transfer interfaces.
Blisters can offer strong unit-dose barrier performance when high-barrier materials are used. Bottles can also work well with good closure control and desiccants, but they are exposed to repeated opening during use.
Confirm shell stability under your handling conditions, confirm fill flow and blend stability, validate that filler settings hold weight within limits across time, and verify that inspection and packaging protect quality through storage and transport.
● FDA: Current Good Manufacturing Practice (CGMP) Regulations. (U.S. Food and Drug Administration)
● eCFR: 21 CFR Part 211 — CGMP for finished pharmaceuticals.
● USP General Chapter <905> Uniformity of Dosage Units (official page).
● ICH Q9 Quality Risk Management (guideline). (database.ich.org)
● ICH Q10 Pharmaceutical Quality System (guideline). (database.ich.org)