Choosing a pharmaceutical machinery manufacturer sounds simple—compare specs, get quotes, pick a name you recognize. In real life, most of the pain shows up later: missed timelines, weak documentation, slow service, and “small” design gaps that turn into big validation and uptime problems. This guide is a practical way to decide who’s truly the best fit for you—whether you’re new to pharma equipment or you’ve bought plenty and just want fewer surprises.
We’ll walk through 10 smart checks you can use to compare any pharma machinery supplier or pharmaceutical equipment manufacturer—from your first shortlist to FAT/SAT and long-term support. First, though, you need one thing that many teams skip.

If you want clean comparisons, you need a clean scope. Otherwise, every supplier “looks good” on paper because you’re not asking the same question in the same way.
Think of this step as turning a fuzzy idea (“we need a line”) into a simple, buyer-friendly URS (User Requirement Specification). It doesn’t need to be formal or scary—just specific enough that two manufacturers would give you comparable answers.
● Product & process reality
Dosage form (tablets, capsules, powders, granules, pellets, liquids), packaging format (blister packs, bottles, stick packs/sachets, cartons), plus material behavior (humidity-sensitive, dusty, sticky, brittle).
● Output and batch rhythm
Target packs/hour, shift pattern, batch sizes, and how often you change formats. High speed means nothing if changeovers eat the day.
● Quality and compliance destination
Where the product will be sold and what standards you must follow (cGMP/GMP expectations, documentation depth, data requirements). This directly affects what a manufacturer must deliver—not just the machine.
● Facility constraints
Footprint, ceiling height, access paths, utilities (power, compressed air, vacuum, cooling), and cleanliness requirements. These are common deal-breakers.
● Automation level and people plan
Do you want “operator-friendly with guardrails” or “engineer-tuned performance”? Who will run it day-to-day, and how comfortable are they with troubleshooting?
● Timeline and integration
Required ship date, installation window, and whether this must integrate with upstream/downstream equipment (for example, blister → cartoner, or counting → capping → labeling).
|
Scope input you define |
What it changes in the quote and design |
|
Dosage form + package format |
Machine type, tooling, sealing method, changeover parts |
|
Target output + batch sizes |
Drive system sizing, buffers, reject logic, OEE expectations |
|
Compliance/document needs |
IQ/OQ readiness, traceability, test protocols, software features |
|
Facility limits (space/utilities) |
Layout, guarding, dust control, power/air specs |
|
Changeover frequency |
Quick-change design, tooling strategy, training intensity |
|
Integration needs |
Line controls, handshakes, conveyors, responsibility boundaries |
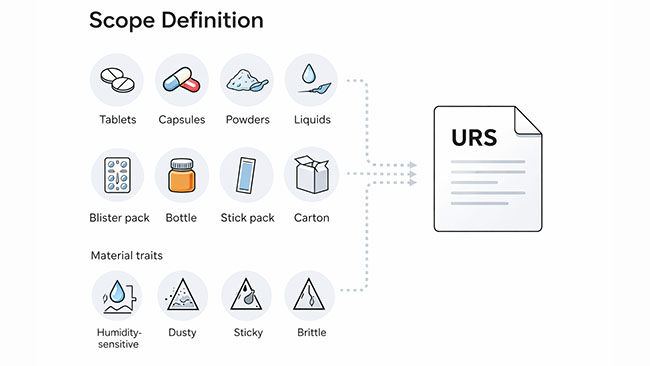
Before you start comparing manufacturers, make sure you can answer these in plain English:
● What product are we running, and what packaging format are we shipping?
● What’s the target output and what’s the typical batch size?
● What are the must-have quality checks and rejects?
● What documentation do we need at handover (basic vs validation-ready)?
● What’s the available space and utilities?
● How often will we change formats, and how fast do we need to change?
● What equipment must this connect to (upstream/downstream), if any?
If you do only one thing before asking for proposals, do this. It makes the rest of the “how to choose a pharmaceutical machinery manufacturer” process faster, more objective, and much harder for weak suppliers to hide behind vague promises.
Next, we’ll use your scope to build a shortlist that’s actually comparable—so you’re not judging apples vs oranges.
Once your scope is clear, your next job is to make sure you’re comparing pharmaceutical machinery manufacturers on the same playing field. This is where a lot of buyers lose time: they collect 6–10 quotes that aren’t even describing the same deliverables.
Here’s a simple way to shortlist any pharmaceutical machinery manufacturer without getting buried in marketing claims:
Start with 6 “proof items” for every supplier
● Similar projects: same dosage form + similar output + similar packaging format.
● Clear scope boundary: what’s included (machine, tooling, conveyors, utilities, installation, training, spares).
● Documentation examples: sample FAT protocol, manuals, parts lists, and (if needed) IQ/OQ templates.
● Service coverage: response time, spare parts availability, and whether they support remote + onsite.
● Factory signals: real production capability (not just an office). Ask for shop-floor photos/video and a simple tour agenda.
● Named references: at least 1–2 customers you can speak to (even if anonymized, you can still verify the story).
Red flags that usually cost money later
● Quotes that avoid specifics: “high quality,” “advanced,” “customizable,” but no test method, no tolerance, no acceptance criteria.
● “Everything is included” with no list of deliverables.
● No clear plan for FAT/SAT, documentation handover, or change control.
● Service promises that are vague: “lifetime support” with no response time or spare parts plan.
A quick reality check: strong manufacturers can usually talk in numbers and processes (headcount, R&D capacity, traceability, outgoing test routines, response time). For example, some established builders openly share details like dedicated R&D teams, audited management systems (ISO + 6S), full procurement traceability, and pre-shipment tests such as extended continuous running. Used lightly, those operational details help you separate real capability from a good website.

“Pharmaceutical equipment manufacturer” is a broad label. The right supplier for a capsule filling machine may not be the best for a high-speed blister packaging machine line integration.
Use category-specific questions. You don’t need 50 of them—just the ones that expose real experience.
● What’s your dosing range and how do you verify fill accuracy?
● How fast is a real changeover (with trained operators, not engineers)?
● What’s the cleaning concept, and what parts are designed to be removed quickly?
● How do you control weight variation and reject out-of-spec tablets?
● What turret/tooling ecosystem do you support, and how fast can you supply wear parts?
● What’s your dust management approach (dedusting, extraction, sealing points)?
● Which forming method fits my product (thermoforming vs cold-forming), and why?
● How do you handle web tracking and registration at speed?
● What’s your sealing integrity check approach (and what’s “pass/fail”)?
● Counting accuracy and reject logic: how do you prove it during FAT?
● How do you handle product changeover without long downtime?
● What’s the approach to dust, static, and fragile products?
● What carton range is proven (not “possible”)?
● Leaflet options, coding/serialization readiness, and jam recovery behavior at speed.
Pros & cons
● Turnkey line from one supplier
◆ Pros: clearer responsibility, fewer interface fights, simpler commissioning.
◆ Cons: you’re betting on one team’s depth across every machine type.
● Best-of-breed machines from multiple suppliers
◆ Pros: higher specialization per machine.
◆ Cons: integration risk goes up (controls, conveyors, timing, ownership of problems).
Certifications help, but what protects you in real audits is evidence: written procedures, traceable parts, validated functions, and controlled documentation.
If you sell into the US, you’ll hear about FDA cGMP expectations (notably 21 CFR Parts 210 and 211). (U.S. Food and Drug Administration)
If you sell into the EU, you’ll hear “EU GMP,” and for equipment qualification/validation, Annex 15 is a common reference point. (Public Health)
Documents worth requesting early (so you don’t chase them later)
● User manuals + maintenance manuals
● Electrical drawings + pneumatic layouts
● Spare parts list + recommended critical spares
● Materials and contact-part declarations (where relevant)
● FAT protocol template + test records example
● Change control / deviation handling approach
l If you need validation readiness: URS mapping, IQ/OQ templates, calibration certificates
If software/data matters (alarms, audit trails, electronic records)
Ask directly whether the pharmaceutical machinery manufacturer can support your expectations around electronic records/e-signatures concepts (often discussed under FDA Part 11 scope and application).
Most projects have some customization—format parts, feeding, dust handling, layout, controls interfaces. The question is whether customization is handled like engineering… or like improvisation.
What “real engineering depth” looks like
● They can show a design review process (even informal).
● They can explain what they won’t customize because it breaks stability or validation.
● They can point to past modifications and what changed in documentation and testing.
Practical questions
● How many engineers support this product line (mechanical + electrical + controls)?
● What is the typical lead time for non-standard parts?
● If a change request happens during build, how is it documented and priced?
(Light benchmark, not a rule): when a supplier can talk openly about sustained R&D investment and dedicated engineering headcount, it’s often a better sign than “custom available” on a brochure.
A machine can be well-built and still fail your project if service is slow or unclear. Treat service like part of the product.
Service basics to clarify
● Installation & commissioning scope (who does what, and what you must provide)
● Training plan (operators + maintenance + documentation handover)
● Spare parts plan (critical spares list, pricing, shipment options)
● Remote support process (video troubleshooting, response time)
● Preventive maintenance program and optional AMC-style support (annual maintenance contract)
If a supplier claims “fast support,” ask for specifics:
● response time target (hours)
● typical time-to-ship for critical spares
● escalation path when production is down
This is the “trust, but verify” step—without turning it into paperwork overload.
Reference checks (keep them simple)
● “What surprised you after installation?”
● “How did they handle the first real breakdown?”
● “Did the documentation match what you needed?”
● “Would you buy from them again?”
Factory visit: what to look for
● Assembly consistency (standard wiring, labeling, cleanliness, organization)
● Incoming material control and traceability mindset
● Testing culture: do they run structured tests or only quick demos?
FAT/SAT: ask for acceptance criteria early
A solid FAT isn’t just “it runs.” It’s “it runs and proves the outcomes.” Many serious suppliers include extended runs because short demos can hide stability issues. For example, Rich Packing provides pre-shipment testing that can include a 24-hour continuous run plus an additional high-load verification window—use it as a benchmark for the level of evidence you ask for, not a box to tick.
 Pre-delivery testing of Rich Packing's pharmaceutical machinery
Pre-delivery testing of Rich Packing's pharmaceutical machinery
The cheapest quote can be the most expensive project if it creates downtime, waste, or constant format headaches.
TCO questions buyers forget to ask
● What are the top wear parts and typical replacement intervals?
● How long is a real changeover with a trained team?
● What’s the expected reject rate during steady production?
● What happens if a key component fails—stocked locally or shipped internationally?
Contract terms that protect you
● Clear deliverables list (documents, spares, tooling, training)
● Warranty scope and exclusions
● Service response expectations (even if it’s not a strict SLA)
● Milestone-based payments tied to tangible acceptance steps
Global purchasing is normal in pharma machinery. Just make sure “global” means more than “we export.”
What to confirm
● Shipping and customs documentation support
● Packing standards and damage claims process
● Remote commissioning capability (if travel is limited)
● Availability of localized service partners (or a proven travel plan)
● Spare parts logistics (stocking plan or fast channels)
When stakeholders disagree, a simple scorecard turns opinions into a decision.
Here’s a lightweight example you can tailor:
|
Category |
Weight |
What “good” looks like |
|
Machine fit (your dosage + output) |
25% |
Proven references + clear acceptance criteria |
|
Documentation readiness |
20% |
Sample FAT + manuals + (if needed) IQ/OQ support |
|
Build quality & standards |
15% |
Transparent components/materials, consistent assembly |
|
Service & spares |
20% |
Clear response process, spares plan, training scope |
|
Commercial & timeline risk |
20% |
Real lead time, clear scope, fair warranty terms |
Score each supplier 1–5, multiply by weight, and the “best for you” usually becomes obvious.
1) How many suppliers should I shortlist?
Usually 3–5. Fewer than 3 limits leverage; more than 5 creates noise.
2) Do I really need a URS?
Yes—even a short one. It prevents apples-to-oranges quotes and protects your timeline.
3) What documents should I request before paying a deposit?
At minimum: scope list, layout/utility requirements, FAT outline, manuals outline, spares list, and delivery timeline.
4) What’s the difference between FAT and SAT?
FAT proves performance at the supplier site; SAT confirms installation and function at your site.
5) How do I judge “GMP compliance” from a machine supplier?
Look for evidence: traceable build practices, documentation discipline, test records, and a validation-ready approach—aligned with the markets you sell into.
6) Is it safer to buy a complete line from one supplier?
Often safer for responsibility and commissioning speed—but only if they have real integration depth.
7) What if I need audit trails or electronic records features?
Bring it up early and confirm what the supplier supports and documents around electronic records expectations.
8) What’s one question that quickly exposes weak suppliers?
“Show me your FAT protocol and a real test record from a similar project.”

So, which supplier is best for you? The answer is usually the one that passes these checks with the least drama: clear scope, proven machine fit, real documentation, disciplined testing, and a service model you can rely on. If you run the process this way, choosing a pharmaceutical machinery manufacturer becomes less about sales talk—and more about evidence, outcomes, and long-term uptime.
2.European Commission: EudraLex Volume 4 (EU GMP Guidelines, incl. Annex 15).
3.FDA: Part 11 — Electronic Records; Electronic Signatures (Scope and Application).
4.PIC/S: Guide to Good Manufacturing Practice for Medicinal Products (PE 009). (picscheme.org)